25+ clinical data management process flow chart
Device determination flow chart. The health domain provides an extremely wide variety of problems that can be.
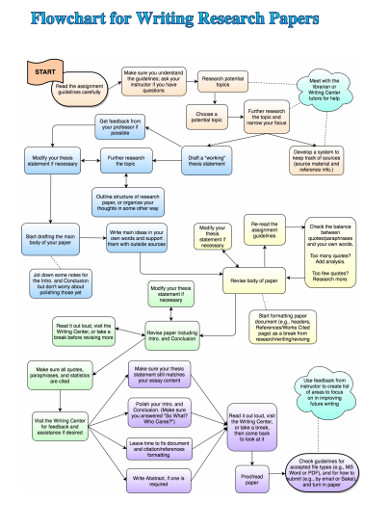
Research Flowchart 18 Examples Format Pdf Examples
Quantity and consistency of data from clinical trials and other sources.
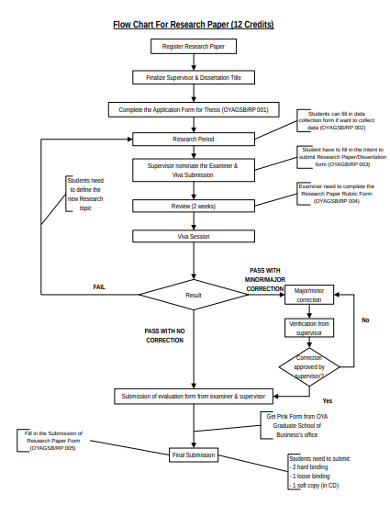
. Clinical calculators 39 3. The PFMEA process needs a complete list of tasks that comprise the. As set forth in ResNo9 the sponsor or hisher CRO should maintain the clinical trial data on file in physical or digital format for a period of five 5 years after the last approval of a request for registration in Brazil.
Data standards are the principal informatics component necessary for information flow through the national health information infrastructure. Vaginal birth after caesarean VBAC Refer to online version destroy printed copies after use Page 3 of 27 Flow chart. Additional relevant studies published through August 2018 during the guideline writing process were also considered by the writing committee and added to the evidence tables when appropriate.
Decision-making framework for women with previous caesarean section. The 2019-CTRules and IND-43 specify that Form CT-04 should be accompanied by one 1 of the following officially mandated fees. A medical device accessory P25 Not a medical device P35.
Duties and responsibilities include formulating policies managing daily operations and planning the use of materials and human resources but are too diverse and general in nature to be classified in any one functional area of management or administration such as personnel. This document provides Harmonised principles of genomic sampling and management of genomic data in clinical studies. 300000 Rupees for Phase I human clinical trials.
Drives or influences the use of a device 40. S451-12 Table 10 reviews these and other racialethnic issues that. Health informatics is the field of science and engineering that aims at developing methods and technologies for the acquisition processing and study of patient data which can come from different sources and modalities such as electronic health records diagnostic test results medical scans.
The FDA reviewed the data for Cefaly through the de-novo pre-market review pathway a regulatory pathway for generally low- to moderate-risk medical devices that are not substantially equivalent to an already legally marketed device ie it. These researchers determined the frequency of ocular OMG in patients referred to an academic neuro-ophthalmologist and determined alternate diagnoses and response to therapy. Plan direct or coordinate the operations of public or private sector organizations.
Acupuncture is a pseudoscience. Process Flow Diagrams PFDs are a graphical way of describing a process its constituent tasks and their sequence. The theories and practices of TCM are not based on scientific knowledge and it has been characterized as quackery.
12713 Process Flow Diagram. They performed a retrospective chart review of patients presenting to the University of Kansas Neuro-Ophthalmology Clinic with suspected OMG over 9 years. Previous vaginal birthstrongest predictor of VBAC.
As per the 2019-CTRules IND-43 and IND-42 a sponsor applicant is responsible for a paying a fee to the Drugs Controller General of India DCGI to submit a clinical trial application. As well as medical device apps becoming a growth area in healthcare management in hospital and in the. Acupuncture is a form of alternative medicine and a component of traditional Chinese medicine TCM in which thin needles are inserted into the body.
Refer to the PANDRH-GCPs for detailed information on electronic trial data systems. There is a range of acupuncture variants which originated in. Bijan Elahi in Safety Risk Management for Medical Devices 2018.
A PFD helps with the brainstorming and communication of the process design. This Guideline will facilitate the implementation of genomic studies by enabling a common understanding of critical parameters for the unbiased collection storage and optimal use of genomic samples and data. Favouring likelihood of VBAC.
With common standards clinical and patient safety systems can share an integrated information infrastructure whereby data are collected and reused for multiple purposes to meet more efficiently the broad scope of data collection and.

Research Flowchart 18 Examples Format Pdf Examples
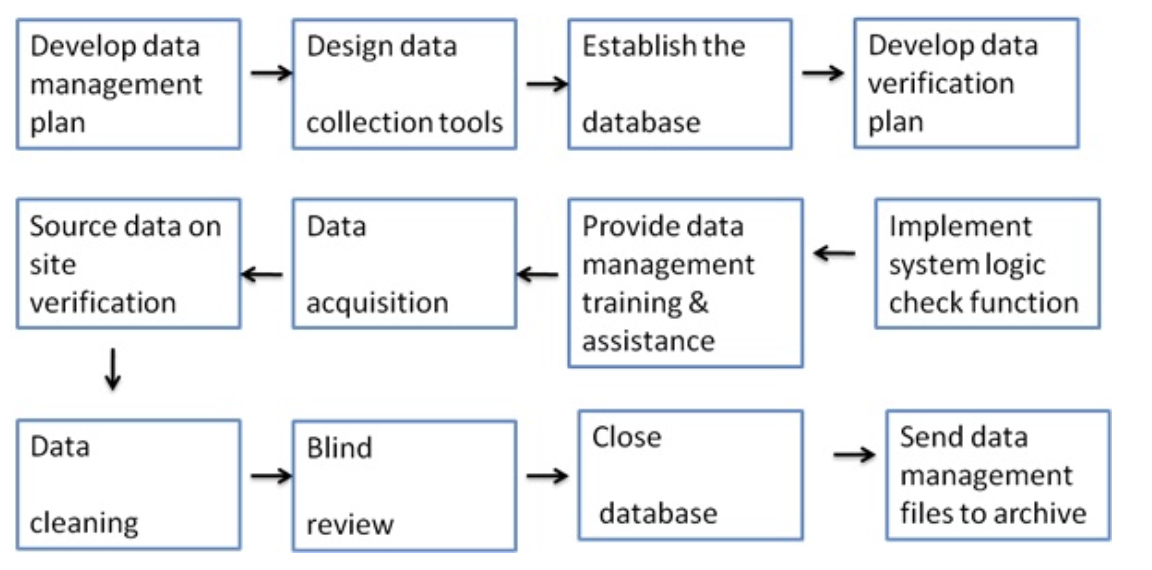
Students Clinical Research Blog Certified Clinical Research Professionals Society Clinical Research Certification
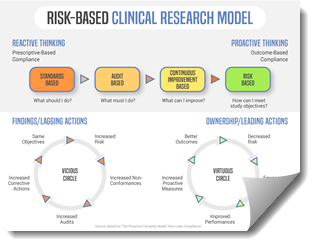
Is Change Management Needed When Going Rbqm Cyntegrity

Pharmacovigilance Clinical Research Blog Certified Clinical Research Professionals Society Clinical Research Certification

Pharmacovigilance Clinical Research Blog Certified Clinical Research Professionals Society Clinical Research Certification
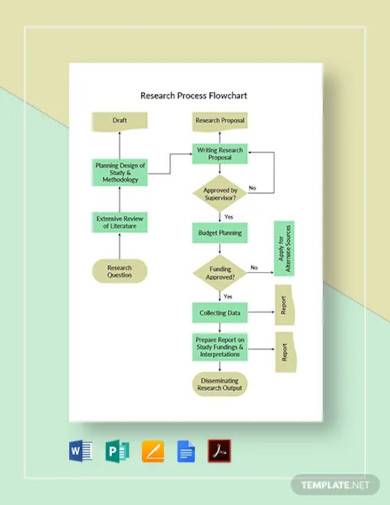
Free 15 Research Flow Chart Samples In Ms Word Pdf

Students Clinical Research Blog Certified Clinical Research Professionals Society Clinical Research Certification
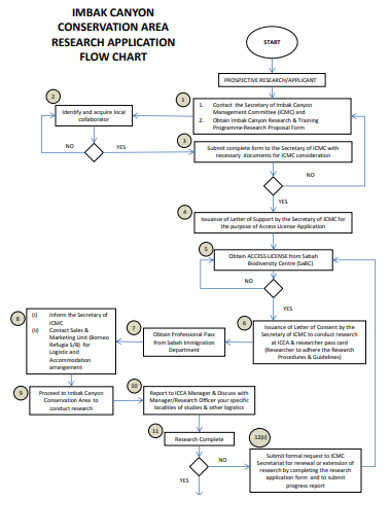
Research Flowchart 18 Examples Format Pdf Examples
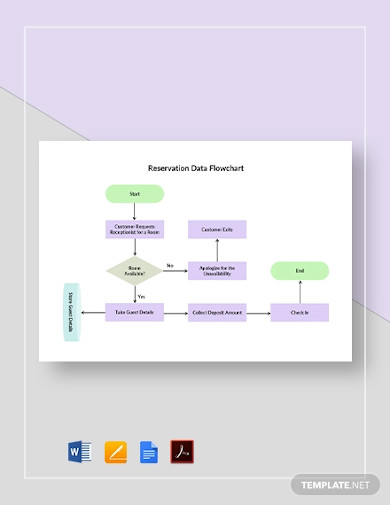
Flow Chart Examples 44 Business Diagram Process Work Examples
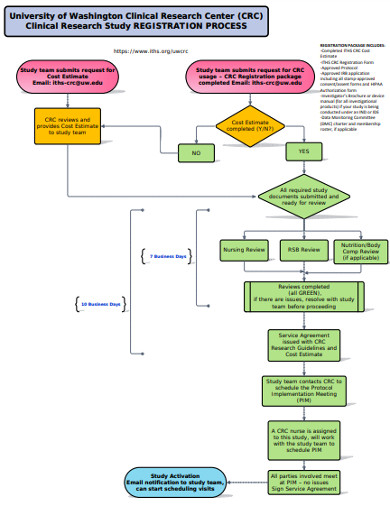
Research Flowchart 18 Examples Format Pdf Examples

The Flowcharts And Workflow Diagrams Of The Medical Algorithms Created By Experienced Doctors And Medical Pro Healthcare Management Workflow Diagram Management
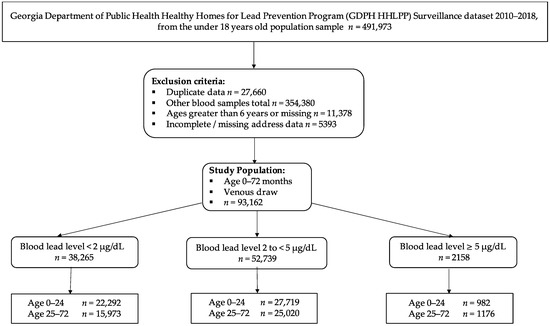
Ijerph Free Full Text Increased Risk Of Sub Clinical Blood Lead Levels In The 20 County Metro Atlanta Georgia Area A Laboratory Surveillance Based Study Html

The Main Concepts Of The Clinical Process Within Continuity Of Care Diagram The Continuity Of Care Is An Or Healthcare Management Workflow Diagram Health Care

Mitchell Halpern Connecticut College Short Hills New Jersey United States Linkedin
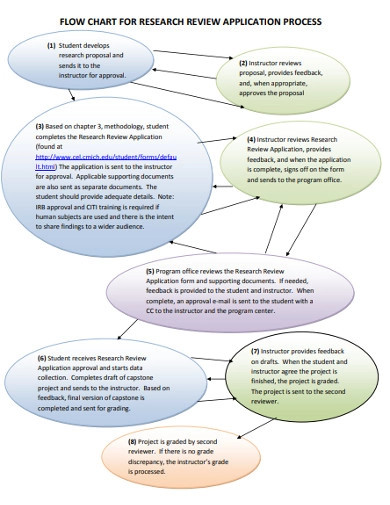
Research Flowchart 18 Examples Format Pdf Examples

Improving The Utilization Of Wearable Device Data Using An Aws Data Lake
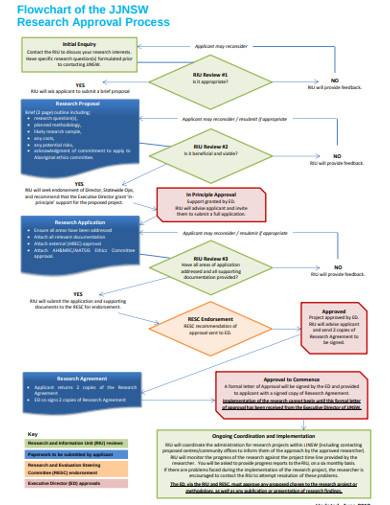
Free 15 Research Flow Chart Samples In Ms Word Pdf